Thousands welcome PBS listing
 There is new hope for cystic fibrosis sufferers as a groundbreaking new drug is added to the PBS.
There is new hope for cystic fibrosis sufferers as a groundbreaking new drug is added to the PBS.
The Federal Government has announced the listing of Trikafta on the Pharmaceutical Benefits Scheme (PBS), which will soon be available for Australians with cystic fibrosis aged 12 years and older, who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. F508del is the most common mutation, affecting about 90 per cent of cystic fibrosis patients.
In Australia, one in 2,500 babies are born with cystic fibrosis and there is currently no cure.
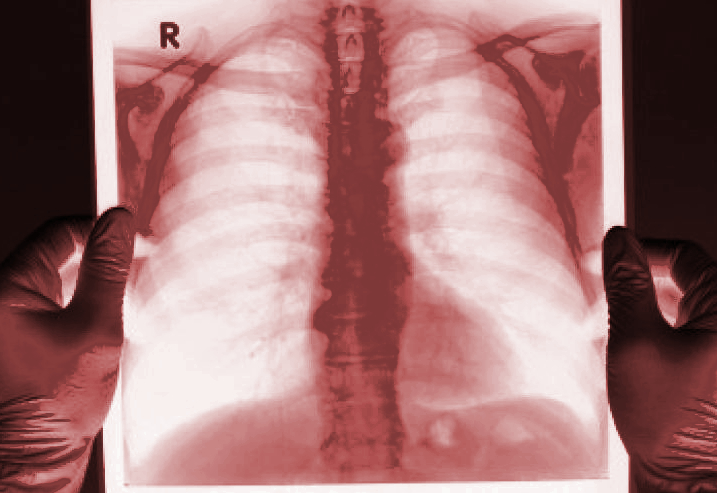
Cystic fibrosis is a progressive, genetic disease that causes persistent lung infections. The median life expectancy for Australians with cystic fibrosis is 47 years.
The condition is caused by genetic defects that limit the flow of chloride and water through cell membranes, resulting in a thick, sticky build-up of mucus in the lungs, pancreas, and other organs and over time limits the ability to breathe and makes it easier for germs to grow.
Trikafta works by improving the flow of chloride and water in patients who have a certain genetic defect and helps improve lung function and breathing.
Health Minister Greg Hunt says the drug produced by Vertex Pharmaceuticals currently costs more than $250,000 a year, but that will drop to just $42.50 when it is added to the PBS register on April 1.







 Print
Print