Working cellular scaffold printed
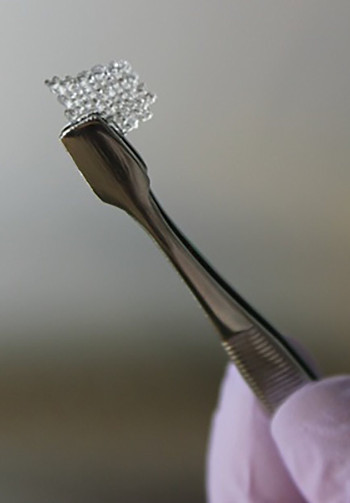 Researchers in the US have 3D-printed a scaffold to repair damaged ovaries.
Researchers in the US have 3D-printed a scaffold to repair damaged ovaries.
A new report in Nature Communications describes the 3D-printed, microporous scaffold that can support the development of mouse follicle cells (egg-producing cells found in ovaries) and can be used to restore ovary function in surgically sterilised mice.
Previous studies have demonstrated the viability of this kind of microporous scaffolds, but the latest work is the first to produce a functional ovarian implant using 3D-printing.
There is a strong clinical need to develop an ovary transplant that can effectively restore fertility and hormones in oncofertility patients — individuals with diminished ovary function due to cancer treatment.
Isolated follicles can be turned into a bioprosthetic ovary, but these cells need to be held in a 3D environment to maintain normal cell-to-cell interactions.
Previous studies using hydrogel scaffolds have enabled mice to give live birth from transplanted bioprosthetic ovaries.
A team at Northwestern University, led by Ramile Shah, has now built on this work, tweaking the design of the scaffold to alter the pore structure, which changes how the follicle and scaffold interact.
They showed that as scaffold interaction increases, follicle spreading is limited and survival increases.
In fact, the authors were able to transplant these bioprosthetic ovaries into surgically sterilised mice. restore fertility, and allow it to give birth to healthy litters of mouse pups.
Although physiologically sufficient to enable pregnancy, the efficiency of ovarian restoration is low and the method is only applicable to mice.
At the moment, the microporous scaffold cannot readily be applied to human follicles, as they are much larger and would require a very different structure and pore size.
Further research is needed to find out whether the scaffold approach could support human follicle survival after implantation.







 Print
Print